BRAVA Breast Fat Graft Study OK by FDA
Posted June 27, 2013 in Breast, Breast LipoStructure, Coleman Technique for Fat Grafting, Dr. Sydney R. Coleman, Fat Grafting, Fat Grafting Research, Minimally Invasive and Non-Invasive Fat Removal
For those who were concerned about the temporary injunction on the study involving Brava use in association with fat grafting there is good news. The FDA has completed its review of the IDE application for the Brava Clinical Study: Breast Reconstruction and Augmentation with the Brave Enhanced Autologous Fat Micro Grafting as of May 3, 2013. In other words, the FDA has granted Brava permission to resume their study, stating: “there are no subject protection concerns that preclude initiation of the investigation,” which is legalese meaning they have found no overwhelming concern to use the Brava device before and after fat grafting to the breasts.
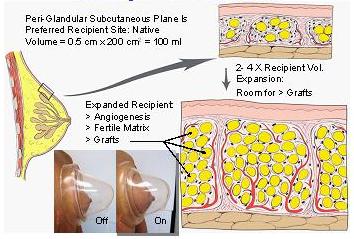
We have certainly found the Brava to be extremely useful in our patients with very small breast “envelopes,” to help develop enough space to graft fat, and for patients who have scarring from prior surgeries. We believe Brava helps prepare the breast to accept more fat, and then appears to help the fat to survive and grow in the first few weeks after surgery.
Should you have any additional concerns, please feel free to contact our office by asking questions on this blog site or one of the following:
- phone (212 571-5200)
- email info@colemancolemanlipostructure.com
- Tribeca Plastic Surgery on FaceBook
For more information on BRAVA, please look at the following links:
- BRAVA Breast Expansion Device Reclassified by FDA
- BREAST AUGMENTATION WITH BRAVA AND COLEMAN FAT GRAFTING Patient Testifies how Coleman Fat Grafting Combined with BRAVA changed her life.
- DR. SYDNEY COLEMAN OF NEW YORK CITY COMBINES THE BRAVA SYSTEM WITH BREAST LIPOSTRUCTURE
© Coleman 2013